近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队在生物气溶胶中关键组分的健康风险评估方面取得最新研究进展,研究成果以《Pseudomonas aeruginosa Activates the NOD-like Receptor Signaling Pathway by Targeting Nasopharyngeal Cells throughout the Whole Respiratory Tract》为题发表在Environment & Health (2025, https://doi.org/10.1021/envhealth.5c00063)期刊上,并被评选为当期补充封面文章。论文的第一作者为博士生彭彩青,通讯作者为安太成教授。

这项工作选取铜绿假单胞菌作为代表性病原细菌,分别人体呼吸道不同部位为研究靶点,即分别以鼻黏膜(HNEpC)、鼻咽(NP69)、支气管(16HBE)及肺(Beas-2B)上皮细胞为暴露实验模型,探究其对人体全呼吸道的不同细胞的暴露评估及其主要的攻击作用靶标部位。作者详细比较了铜绿假单胞菌对各呼吸道不同部位上皮细胞系的细胞毒性机制,提出了铜绿假单胞菌主要是通过激活炎症相关信号通路引发呼吸道疾病的机制。这项研究评估的健康风险适用于医疗场所、污水处理设施及潮湿密闭建筑等生物气溶胶高暴露风险场景,为病原体的呼吸健康风险评估提供了一定的直接证据,对研究革兰氏阴性菌及其类似的病原体的环境暴露与健康效应具有一定的方法学借鉴价值。
论文的网址: https://pubs.acs.org/doi/pdf/10.1021/envhealth.5c00063
论文补充封面:
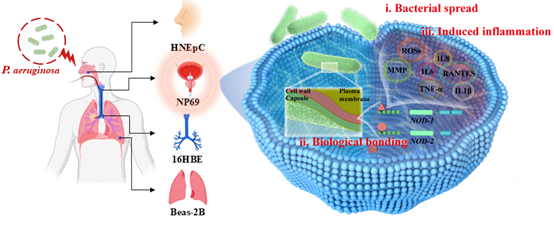
生物气溶胶广泛存在于空气中,包含其中的细菌暴露可能会引发过敏反应和呼吸道疾病。然而,致病菌影响呼吸道健康的具体机制,及其在呼吸道中不同部位主要靶细胞的作用机理尚不明确。本研究分别系统探讨了铜绿假单胞菌对人体呼吸道不同部位的上皮细胞系(鼻黏膜HNEpC、鼻咽NP69、支气管16HBE、肺Beas-2B)的细胞毒性。通过追踪铜绿假单胞菌暴露全过程,发现NP69细胞为人体呼吸道中主要的可能攻击靶细胞靶点,而HNEpC细胞在四种呼吸道细胞中敏感性最低。铜绿假单胞菌可从NP69细胞外进入胞内,伴随NOD2(一种特异性结合细菌细胞壁的RNA分子)表达显著上调(1.5-26.5倍)。此外,细菌进入NP69细胞后,细胞增殖活性下降6.8%-46.1%,炎症细胞因子白细胞介素IL-6和IL-1β水平分别升高14.5%-181.0%和50.2%-238.2%。上皮-间充质转化(EMT)标志物在基因和蛋白水平的表达变化及迁移能力增强的功能表型,证实铜绿假单胞菌可通过激活NOD样受体信号通路诱导上皮细胞恶性转化。本研究全面考察了大气生物气溶胶中的细菌对人呼吸道各部位不同细胞系的细胞毒性比较,阐明了对呼吸道不同特定部位细胞系毒性作用及其损伤机制,为大气生物气溶胶的健康风险评估、风险预防及气载细菌的防控提供了重要科学见解和理论依据。
图文摘要:
论文英文摘要:
Bioaerosols are commonly present in air, and exposure of bacteria in bioaerosols may lead to allergic reactions and respiratory diseases. However, precise mechanisms underlying the impact of pathogenic bacteria on respiratory health remain poorly understood and their primary target cells in respiratory tract have not been identified. We systematically explored cytotoxicity of Pseudomonas aeruginosa (P. aeruginosa) on the whole epithelial cell lines representing different segments of the respiratory tract, including HNEpC (nasal mucosa), NP69 (nasopharynx), 16HBE (bronchus), and Beas-2B (lung) cells. By tracing entire process of exposure to P. aeruginosa, NP69 cells emerged as the primary target, with HNEpC cells showing the lowest susceptibility among the four respiratory cell types. P. aeruginosa were transmitted from extracellular space to intracellular region of NP69 cells, accompanied by significant up-regulation (1.5−26.5 fold) of NOD2 expression, an RNA molecule that specifically binds to bacterial cell walls. Moreover, cell proliferation activity decreased by 6.8%–46.1%, while levels of inflammatory cytokine interleukin IL-6 and IL-1β increased by 14.5%−181.0% and 50.2%−238.2%, respectively, following intracellular transition of bacteria in NP69 cells. The changes in epithelial-mesenchymal transition (EMT) marker expression at genetic and protein level, and enhancement of functional phenotypes (migratory capacity) confirmed potential of P. aeruginosa to induce malignant transformation of epithelial cells by activating the NOD-like receptor signaling pathway. This comprehensive examination of comparative cytotoxicity of bacteria on cells throughout the human respiratory tract elucidates specific target cells and damage mechanisms, offering valuable insights into health risk assessment, risk prevention, and bacteria control.
项目资助:本研究得到国家重点研发项目(2023YFC3708204)和国家自然科学基金(U1901210 和 42177410)的大力支持。