近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队题为《The association between estrogenic activity evolution and the formation of different products during the photochemical transformation of parabens in water》的学术论文在Water Research, 2025, 276: 123236杂志上发表。论文的第一作者为博士生牛笑林,通讯作者为安太成教授。论文的合作者还包括中国科学院生态环境研究中心的马梅研究员和兰州大学应用有机化学国家重点实验室的惠新平教授。该研究主要关注水环境中典型防腐剂系列对羟基苯甲酸酯在光化学转化过程中的环境行为及其雌激素活性的演变,揭示了典型防腐剂烷基链长、转化产物与毒性演变之间的相关性。这项研究结果深化了对药品和个人护理产品(PPCPs)等新污染物在环境中的迁移转化行为及其潜在健康风险的理解,为评估其对生态环境及人类健康的影响提供了重要的科学依据。

论文DOI:https://doi.org/10.1016/j.watres.2025.123236
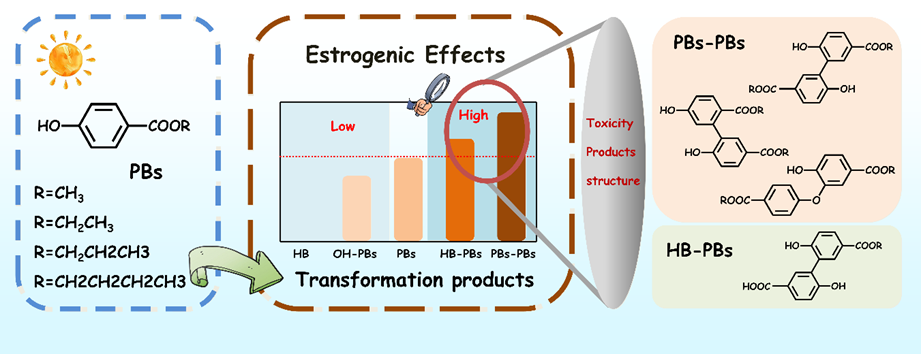
光化学转化是影响药物和个人护理品在水生生态系统中环境归趋的重要因素。目前,针对毒性演变与转化产物的相关性研究却鲜有报道。本研究聚焦水环境中典型防腐剂系列对羟基苯甲酸酯(PBs)在光化学转化过程中的环境行为及其雌激素活性的演变,重点关注侧链烷基链长的影响(对羟基苯甲酸甲酯(MPB),对羟基苯甲酸乙酯(EPB),对羟基苯甲酸丙酯(PPB)和对羟基苯甲酸丁酯(BPB))。基于MS/MS分析结果表明:系列PBs光化学转化过程中主要形成四类转化产物:(1)对羟基苯甲酸(HB),已被证明没有雌激素活性;(2)羟基化产物(OH-PBs);(3)HB和PBs之间形成的二聚体产物(HB-PBs);(4)由两分子相同的PBs形成的二聚体产物(PBs-PBs),含三种不同的同分异构体。由于缺少标品,作者自行制备合成了一系列产物OH-PBs,并采用酵母双杂交报告实验首次测定了产物OH-PBs的EC50值,分别为OH-MPB(< 1×10-3M)、OH-EPB(2.05×10-4 M),OH-PPB(5.05×10-5M)、OH-BPB(1.89×10-5M)。对应的母体化合物的EC50值分别为MPB(3.93×10-4 M)、EPB(4.43×10-5M)、PPB(5.19×10-6M)、BPB(1.68×10-6M)。表明这种OH-PBs产物的雌激素活性比对应的母体化合物低一个数量级。相比之下,理论计算评估产物HB-PBs及含三种同分异构体的PBs-PBs的雌激素活性均明显高于母体化合物,分别提高了9-14倍和32-184倍。在产物PBs-PBs的三种同分异构体中,醚类二聚体的雌激素活性最高,其次是联苯类二聚体。同时发现随着侧链烷基链长的增加,产物OH-PBs的雌激素效应也随之增加,这与母体化合物中观察到的规律一致。同时发现光化学转化过程中,MPB和EPB的雌激素活性总体呈下降趋势,而PPB和BPB的雌激素活性在初期保持稳定后迅速下降,这与形成各种毒性产物的贡献密切相关。以上结果阐明了典型系列防腐剂分子结构、转化产物和雌激素活性之间的相关性,同时也强调PBs光化学转化过程中雌激素活性演变的重要性。
图片摘要:
论文的英文摘要附如下:
ABSTRACT:
Photochemical transformation is a critical factor influencing the environmental fate of pharmaceutical and personal care products in aquatic ecosystems. However, the relationship between toxicity evolution and the formation of various transformation products has been seldom explored. This study investigates the behavior and changes in estrogenic activity during the photochemical transformation of a series of typical endocrine-disrupting parabens (PBs), focusing on the effects of increasing alkyl-chain length (MPB, EPB, PPB and BPB). Based on MS/MS analysis, four types of transformation products were identified: (1) p-hydroxybenzoic acid (HB), which exhibits no estrogenic activity; (2) hydroxylated products (OH-PBs); (3) dimer products formed between HB and PBs (HB-PBs); and (4) dimer products formed from identical PBs (PBs-PBs), comprising three distinct isomers. In the absence of standard sample, OH-PBs were synthesized and their estrogenic activity was evaluated using a yeast two-hybrid reporter assay. The EC50 values were determined to be <1×10-3 M for OH-MPB, 2.05×10-4 M for OH-EPB, 5.05×10-5 M for OH-PPB, and 1.89×10-5 M for OH-BPB. These indicate that the estrogenic activity of OH-PBs is one order of magnitude lower than that of the corresponding PBs. Both HB-PBs and the three isomers of PBs-PBs exhibited significantly higher estrogenic activities than their corresponding parent compounds, increasing 9 – 14 and 32 − 184 times, respectively, based on theoretical calculations. Among the three PBs-PBs isomers, the highest estrogenic activity was observed in the ether dimer, followed by the biphenyl dimers. Consistent with the parent compounds, the estrogenic activities of OH-PBs, HB-PBs, and PBs-PBs increased with the length of the alkyl-chain. The estrogenic activity of MPB and EPB followed an overall downward trend during the photochemical transformation, whereas PPB and BPB remained stable initially before declining rapidly. This behavior was associated with the contributions of toxic transformation products. These findings elucidate the relationship between molecular structure, transformation products, and estrogenic activity, highlighting the importance of understanding estrogenic activity evolution during the photochemical transformation of PBs.
项目致谢:本研究得到国家重点研发计划项目(资助号:2022YFC3105600)、国家自然科学基金项目(资助号:42322704、41977365、42277222)和广东省基础与应用基础研究基金项目(资助号:2023B1515020078)的共同资助。