近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队在国际期刊Science of the Total Environment (2023, 859, 160311, http://dx.doi.org/10.1016/j.scitotenv.2022.160311) 上发表题为《A new bi-radical species formed during the photochemical degradation of synthetic musk tonalide in water: Study of in-situ laser flash photolysis and validation of synthesized standard sample》的研究论文。论文的第一作者为博士生罗娜,通讯作者为安太成教授。

论文网址:http://dx.doi.org/10.1016/j.scitotenv.2022.160311
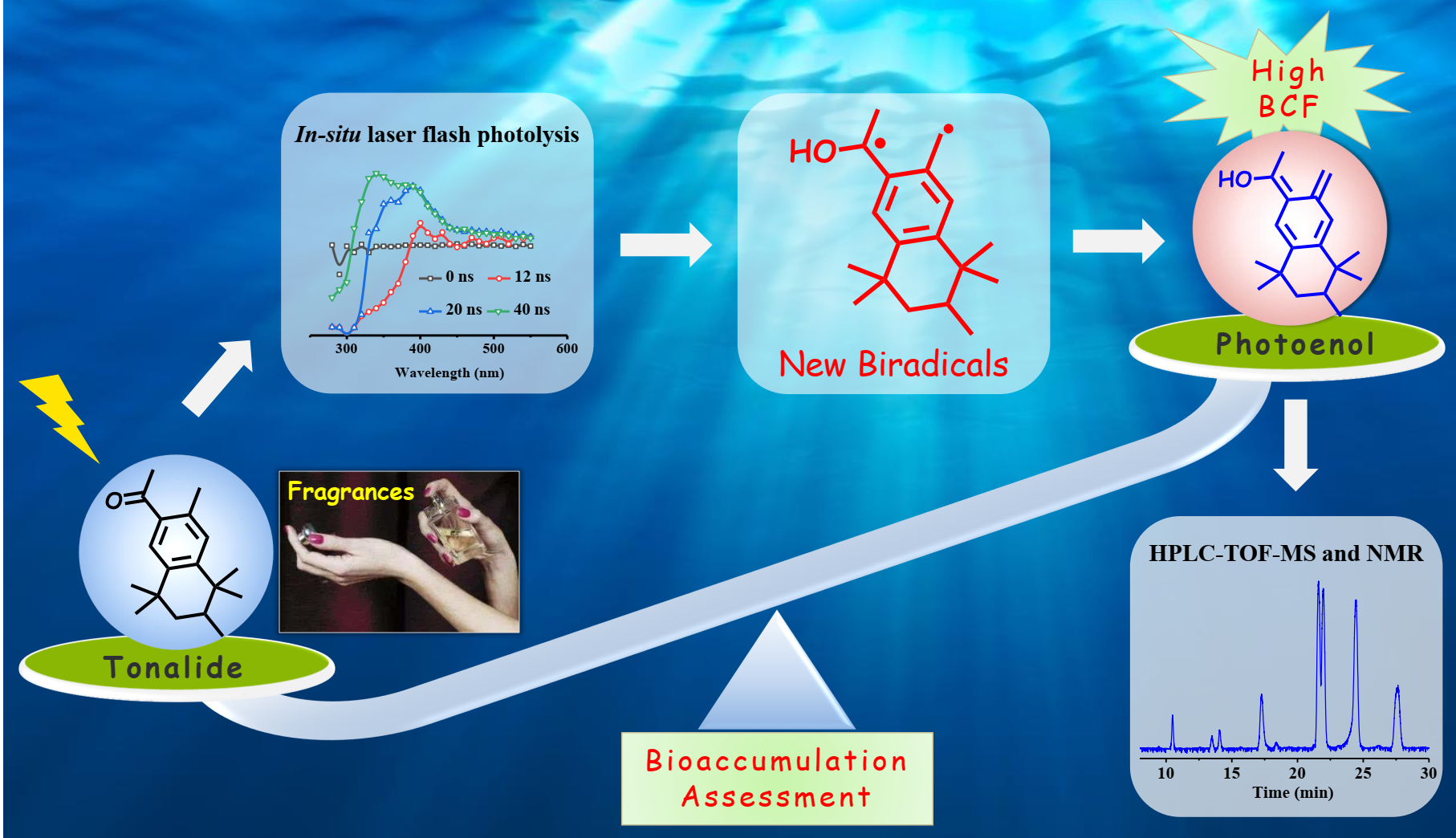
新兴有机污染物(EOCs)及其光化学降解产物在水体中广泛存在,其环境行为以及对人类健康的不良影响引起了更多环境科学工作者的关注。合成麝香作为一类典型的EOCs,已被广泛应用于各种化学护理消费品中。本研究以典型吐纳麝香为合成麝香(Tonalide)的代表,研究了其在水体中紫外光照射下的转化机理和途径。结果表明:一个关键的双自由基作为初始活性物种,主导了吐纳麝香的快速光化学降解。首先采用原位激光闪光光解技术在340 nm处观察到双自由基的典型吸收峰,发现其绝对速率常数为3.61±0.01 × 109 M−1 s−1,寿命为83.3 ns。进一步通过高效液相色谱-三重四极杆-飞行时间质谱联用仪鉴定了吐纳麝香的光化学降解中间产物,并通过制备液相色谱分离制备了部分关键降解中间产物的标准样品,并经核磁共振质谱验证了关键降解中间产物的精确结构。在此基础上,提出了吐纳麝香光化学降解机理为光化学原位形成的双自由基持续进行光烯醇化及其与水体中的O2进一步发生环加成反应。此外还发现双自由基光烯醇化反应生成的主要降解中间产物的生物富集性显著高于原化合物吐纳麝香,中间产物Photoenol的生物富集因子甚至是原化合物的13倍。本研究的创新在于吐纳麝香光化学降解中首次发现了一种新的双自由基中间产物,可以更好地阐释合成麝香在水环境中的迁移转化行为为环境归趋研究提供一定的数据支撑。
图文摘要:
英文摘要:
The ubiquitous presence of synthetic musks is causing serious concern due to their transformation species and environmental impacts. In this study, tonalide (AHTN) was selected as a model of synthetic musks to find out the transformation mechanism and pathway in water under UV irradiation. Results showed that AHTN could undergo rapid photochemical degradation via a new pivotal biradical as the initial active species. The biradicals with a typical absorption peak at 340 nm was observed by in-situ laser flash photolysis technology, and the absolute decay rate constant was obtained as 3.61 ± 0.01×109 M-1s-1 with the life-time of 83.3 ns. The photochemical degradation by-products of AHTN were also identified by high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry, and the precise structures of key by-products have been validated by our preparative synthesized standard samples confirmed by nuclear magnetic resonance. Thus, the mechanism of AHTN photochemical degradation, continuous photoenolization of the biradicals and followed cycloaddition reaction with O2, was proposed as the predominant pathway. The main degradation by-product, photoenol which has a higher bioconcentration factors than that of AHTN, were found to form from the biradicals photoenolization. This study is the first work to propose a new biradical as the photoenol precursors during photochemical degradation of AHTN in water.