近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队题为《Elucidating the critical oligomeric steps in secondary organic aerosol and brown carbon formation》的学术论文在大气化学与物理领域期刊Atmospheric Chemistry and Physics (IF2020=6.133)上发表。论文第一作者为姬越蒙教授,第二作者为石秋菊博士生,通讯作者为安太成教授和姬越蒙教授。

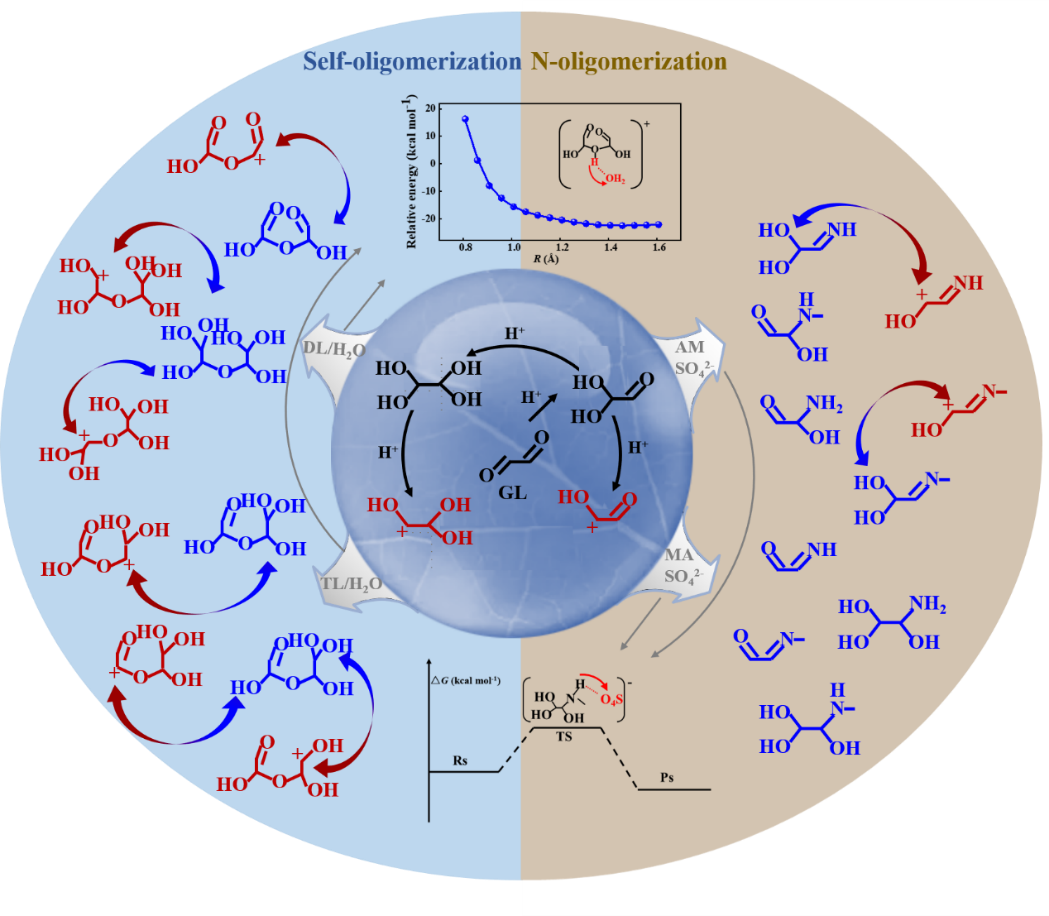
小分子α-二羰基化合物是大气中二次有机气溶胶(SOA)和棕碳(BrC)的重要前体物,然而其形成SOA和BrC的化学机理仍存在较多不清晰之处。本论文主要通过量子化学和动力学速率计算结合的手段,阐述了乙二醛(GL)液相低聚反应的动力学及机理,分别探明了在没有或有甲胺/氨气(MA/AM)参与时GL液相低聚形成SOA和BrC的关键步骤。我们的结果表明:乙二醛存在如下几个异构化过程:质子化产生二醇/四醇及碳正离子、二醇/四醇及MA/AM亲核加成到碳正离子上、脱质子化形成并衍生低聚物及含氮杂环。质子化和亲核加成的化学反应过程均无能垒,其主要通过电荷吸引介导。当溶液中不存在MA/AM时,脱质子化过程通过水分子以无能垒过程介导;但是当溶液中存在MA/AM时,脱质子化通过酸根离子催化进行,是含氮碳正离子后续反应产生含氮杂环的速率决速步骤。碳正离子介导的GL非均相反应速率为4.62 × 10-3s-1,在城市环境下的气溶胶生长率为1.41 μg m-3h-1,与Liggio等人的实验结果(1.44 μg m-3 h-1)一致。我们的结果为准确评估小分子α-二羰基化合物对SOA及BrC形成的作用提供了基本的微观化学反应机理及动力学数据。
论文网址:https://acp.copernicus.org/articles/22/7259/2022/acp-22-7259-2022.pdf
图文摘要:
论文英文摘要
ABSTRACT
Small α-dicarbonyls represent the major precursors of secondary organic aerosol (SOA) and brown carbon (BrC) in the atmosphere, but the chemical mechanisms leading to their formation remain unclear. Here we elucidate the fundamental kinetics and mechanisms for aqueous-phase oligomerization of glyoxal (GL) using quantum chemical and kinetic rate calculations. Our results identify several essential isomeric processes for GL, including protonation to yield diol/tetrol and carbenium ions, nucleophilic addition of carbenium ions to diol/tetrol as well as to free methylamine/ammonia (MA/AM), and deprotonation to propagate oligomers and N-heterocycles. Both protonation and nucleophilic addition occur without activation barriers and are dominantly driven by electrostatic attraction. Deprotonation proceeds readily via water molecules in the absence of MA/AM but corresponds to the rate-limiting step for N-containing cationic intermediates to yield N-heterocycles. On the other hand, the latter occurs readily via a catalytic process by acidic anions (e.g., SO42-). A carbenium ion-mediated reaction rate of GL is 4.62 × 10-3s-1under atmospheric conditions, in good agreement with the experimental data. Our results provide essential mechanistic and kinetic data for accurate assessment of the role of small α-dicarbonyls in SOA and BrC formation.