近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队题为《Formation kinetics and mechanisms of ozone and secondary organic aerosols from photochemical oxidation of different aromatic hydrocarbons: dependence on NOx and organic substituent》的学术论文在大气化学与物理领域的顶级期刊Atmospheric Chemistry and Physics(IF=5.414)杂志上发表。论文的第一作者为博士研究生罗灏和陈江耀教授,通讯作者为安太成教授。
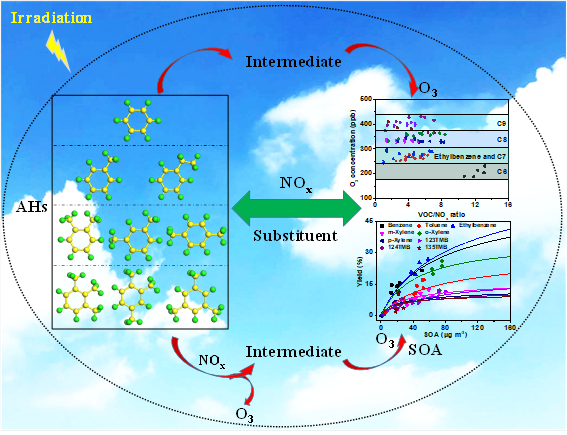
芳香烃(AHs)是大气环境中臭氧(O3)和二次有机气溶胶(SOA)的重要前体物,直接或间接威胁空气质量和公众健康。大气中的AHs主要来自人为源,如固定源燃烧、道路交通、溶剂产品使用和工业过程等,通常由1个苯环和数量小于4个的甲基(例如甲苯、二甲苯、三甲苯)或乙基(例如乙苯)组成。研究发现,这些AHs的光化学氧化对反应条件较为敏感(例如VOC/NOx比率),最终影响AHs氧化生成O3和SOA的动力学和机理。早期已经有相关实验模拟研究证实了相对较高的VOC/NOx比率对SOA产率有抑制作用,而VOC/NOx比率也可能影响O3的形成。从野外观测结果来看,大气中VOC/NOx的实际比值总是随着季节的变化而变化。尽管VOC/NOx比率对AHs光化学氧化生成O3和SOA的影响具有重要的环境意义,但目前的研究主要集中在NOx浓度的变化上。而有关AHs含量及取代基对O3和SOA形成动力学及机理的影响尚不清楚。因此,考虑到真实大气的复杂性,作者在实验室烟雾箱中有效模拟了不同VOC/NOx比率下的大气光化学反应,探索不同结构和浓度AHs中O3和SOA的形成动力学和机理是非常必要的。
本文选择了9种不同取代基数目和取代位置的AHs(苯、甲苯、乙苯、间二甲苯、邻二甲苯、对二甲苯、123-三甲苯、124-三甲苯、135-三甲苯),在室内烟雾箱系统中系统开展了它们的光化学氧化行为研究,比较了它们在有无NOx存在情况下形成O3和SOA的动力学和机理。研究结果发现:AHs直接光解只产生少量O3,且AHs取代基数越少,O3浓度越高。NOx的加入显著促进了O3和SOA的生成。这是因为NO2的加速转化以及NO与AHs反应的协同效应有利于O3和挥发性中间产物的形成。同时发现提高VOC/NOx比值中AHs的浓度进一步提高了O3和SOA的生成速率和浓度,这可能揭示了VOCs的浓度增加可以提高O3和SOA的生成量。此外,随着AHs取代基数目的增加,O3的生成进一步增强,但受取代基位置的影响可以忽略。不同的是,SOA产率随AHs取代基数的增加而降低,但是邻甲基取代AHs促进了SOA的生成。模型拟合和在线中间产物分析结果一致证实:苯环上取代基数目的增加能够有效抑制二羰基中间体的生成,但二羰基中间产物优先由邻甲基取代的AHs氧化生成,导致SOA产率的变化趋势不同。此外,苯环上取代基数目的增加对低聚反应的抑制是SOA产率降低的另一个主要原因。本论文得到的这些结果有利于科学人员更加清楚地认识了NOx和有机分子结构对AHs光化学氧化生成O3和SOA的影响,为研究AHs在实际大气环境中转化为二次污染物提供了坚实的实验依据。
Graphical Abstract
论文链接: https://doi.org/10.5194/acp-21-7567-2021
论文英文摘要:
Aromatic hydrocarbons (AHs) contribute significantly to ozone and secondary organic aerosol (SOA) formation in atmosphere, but formation mechanisms are still unclear. Herein, photochemical oxidation of nine AHs was investigated in chamber. Only small amount of ozone was produced from direct photochemical oxidation of AHs, while fewer AH substituent number resulted in higher concentrated ozone. Addition of NOx increased ozone and SOA production. Synergetic effect of accelerated NO2conversion and NO reaction with AHs boosted ozone and volatile intermediate formation. Promoting AH concentration in VOC/NOx ratio further increased formation rates and concentrations of both ozone and SOA. Additionally, ozone formation was enhanced with increasing AH’s substituent number but negligibly affected by their substituent position. Differently, SOA yield decreased with increased substituent number of AHs, but increased with ortho methyl group substituted AHs. Model fitting and intermediate consistently confirmed that increasing substituent number on phenyl ring inhibited generating dicarbonyl intermediates, which however were preferentially produced from oxidation of ortho methyl group substituted AHs, resulting in different changing trend of SOA yield. The restrained oligomerization by increased substituent number was another main cause for decreased SOA yield. These results are helpful to understand photochemical transformation of AHs to secondary pollutants in real atmosphere.