近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队在大气中一氧化二氯( Cl2O )来源方面取得重要进展。研究成果以“Promoting Cl2O Generation from the HOCl + HOCl Reaction on Aqueous/Frozen Air‒Water Interfaces”为题发表在Journal of the American Chemical Society (2024,146(46): 31935-31944; https://pubs.acs.org/doi/10.1021/jacs.4c11337)期刊上。论文主要作者为我院张维娜副教授和硕士生郑达远等,通讯作者为安太成教授和美国宾夕法尼亚大学Joseph S. Francisco教授。
论文网址:https://pubs.acs.org/doi/10.1021/jacs.4c11337
中文摘要:
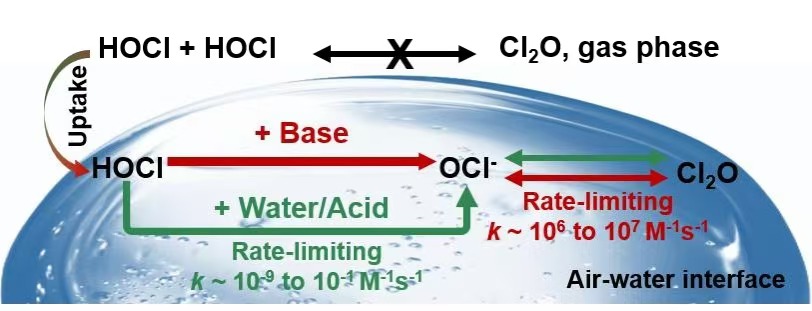
本文通过从头算分子动力学(AIMD)计算,系统揭示了在液相/冰冻层空气-水界面上次氯酸(HOCl)自反应生成一氧化二氯(Cl2O)的过程。研究发现:在无催化剂条件下,HOCl在液相/冰冻层空气-水界面上的自反应速率常数虽高于气相,但反应仍难以发生。因此,本研究使用水、硝酸、氨、甲胺和二甲胺作为催化剂,分别系统研究了它们对于液相/冰冻层空气-水界面上HOCl自反应的促进作用。研究发现:与气相中HOCl一步自反应即可生成Cl2O的机制不同,液相/冰冻层空气-水界面上的Cl2O生成涉及两个基本步骤:i)一个是HOCl脱质子化反应,产生ClO-,ii)另一个是HOCl被ClO-提取Cl-的反应,生成Cl2O。此外,还发现在引入水、硝酸、氨、甲胺和二甲胺作为催化剂后,由于HOCl的第一步反应脱质子化步骤被明显加快,因此Cl2O的生成速率得到显著提高。特别是在碱催化条件下,Cl2O的生成效率较其他条件具有大幅提升。由此研究表明:在极地平流层和低层对流层大气中,HOCl在液相/冰冻层空气-水界面上的自反应是大气中Cl2O的一个潜在来源。
图文摘要:
英文摘要:
Hypochlorous acid (HOCl) is considered a temporary reservoir of dichlorine monoxide (Cl2O). Previous studies have suggested that Cl2O is difficult to generate from the reaction of HOCl + HOCl in the gas phase. Here, we demonstrate that Cl2O can be generated from the HOCl + HOCl reaction at aqueous/frozen air–water interfaces, which is confirmed by ab initio molecular dynamic calculations. Distinct from the one-step reaction in the gas phase, our results show that Cl2O generation from HOCl + HOCl on aqueous/frozen interfaces involves two elementary steps, namely, one HOCl deprotonation and one Cl- abstraction from the other HOCl. Specifically, the mechanisms of neutral/acidic catalysis from interfacial water/nitric acid and base catalysis from ammonia, methylamine and dimethylamine have been examined. For the former, HOCl deprotonation is the rate-limiting step, and the total k of Cl2O generation increases to 9.23 × 10-9 ~ 9.10 × 10-1 M-1s-1 at the aqueous interface and 3.20 × 10-7 ~ 4.10 × 10-3 M-1s-1 at the frozen interface, which is at least 23 and 25 orders of magnitude greater than that of gaseous k (3.31 × 10-32 M-1s-1). For the latter, the rate-limiting step is changed to Cl- abstraction, whose total k dramatically increases to 1.40 ~ 8.97 × 107 M-1s-1 at the aqueous interface and 7.12 ~ 9.99 × 106 M-1s-1 at the frozen interface. Interestingly, the Cl2O production rates ranked in the order of dimethylamine<methylamine<ammonia and decreased with increasing catalytic alkalinity. These findings provide new insights for understanding other Cl2O sources beyond the ClONO2 + HOCl reaction.
资助项目:本研究受到国家自然科学基金(42020104001、42277081和42077189)、广东省基础研究和应用基础研究基金项目(2024A1515012691和2019B151502064)、广东省重点领域研发计划(2022-GDUT-A0007)和广州市基础与应用基础研究项目(202201010496)的联合资助。