《OH-initiated oxidation of acetylacetone: Implications for ozone and secondary organic aerosol formation》
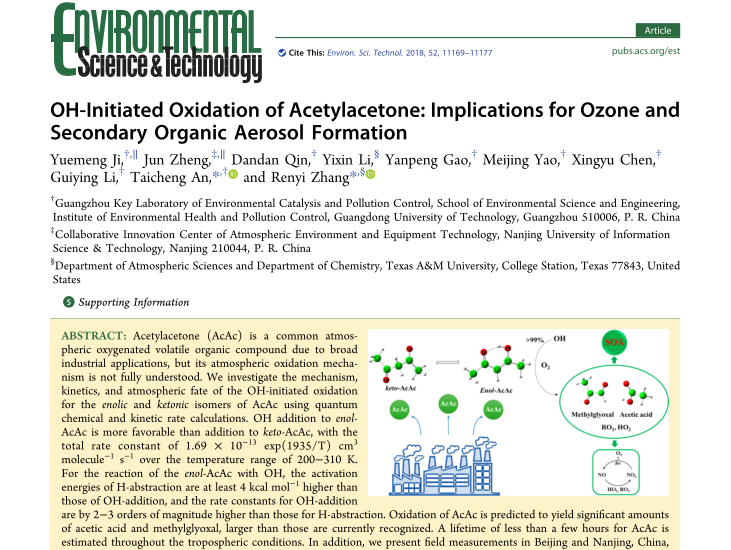
Abstract: Acetylacetone (AcAc) is a common atmospheric oxygenated volatile organic compound (OVOC) due to broad industrial applications, but its atmospheric oxidation mechanism is not fully understood. Here we investigate the mechanism, kinetics, and atmospheric fate of the OH-initiated oxidation for the enolic and ketonic isomers of AcAc using quantum chemical and kinetic rate calculations. Because of the conjugated p-electron effect, OH addition to enol-AcAc is more favorable than addition to keto-AcAc, with the total rate constant of 1.69 × 10-13 exp(1935/T) cm3 molecule-1 s-1 over the temperature range of 200-310 K. For the reaction of the enol-AcAc with OH, the activation energies of H-abstraction are at least 2 kcal mol-1 higher than those of OH-addition, and the rate constants for OH-addition are by 2‒3 orders of magnitude higher than those for H-abstraction. We demonstrate that oxidation of AcAc yields significant formation of acetic acid and methylglyoxal, which is larger than is currently recognized. Using the predicted temperature-dependence kinetic data, a lifetime of less than a few hours for AcAc is estimated throughout the tropospheric conditions. In addition, we present field measurements of the concentration of AcAc in Nanjing, China, reaching as high as a few parts per billion (ppb). Our results reveal that the OH-initiated oxidation of AcAc contributes importantly to ozone and SOA formation under polluted environments and provide the key kinetic and mechanistic data for its inclusion in atmospheric models.
Download Address:https://pubs.acs.org/journal/esthag/, IF2016=6.653